Answer
CH₂
Step-by-step explanation
It is important to note that you are dealing with a hydrocarbon, that is, a compound that contains only carbon and hydrogen.
Since the products of this combustion reaction are carbon dioxide, CO2, and water, H2O, this let you know that all the carbon that was initially a part of the hydrocarbon will now be part of the carbon dioxide. Likewise, all the hydrogen that was initially a part of the hydrocarbon is now a part of the water.
To determine how many moles of carbon and of hydrogen were originally present in the hdyrocarbon, this means that you can use the number of moles of water and carbon dioxide, respectively, as follows.
For water
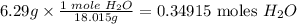
For CO2
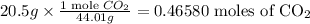
Every mole of water contains two moles of H and one mole of O. This implies which means that the reaction produced
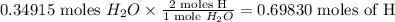
Every mole of carbon dioxide contains 1 mole of carbon and 2 moles of oxygen, it follows that the reaction also produced
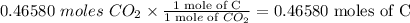
Lastly, to find the mole ratio that exists between carbon and hydrogen in the hydrocarbon, divide the mole values by the smallest one.

The empirical formula of the hydrocarbon will be C₁H₂ ⇒ CH₂
.