Given:
The weight of 18-month-old girls is normally distributed with a mean of 24.8 pounds and a standard deviation of 2.9 pounds. The table of z-score percentile is given.
Required:
Find the percentile of the 18-month-old girl whose weight is between 19.1 and 23.6 pounds.
Step-by-step explanation:
First, find the z-score by using the formula:
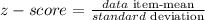
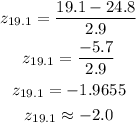
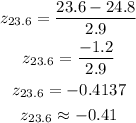
Now by using the table find the percentile corresponding to the z-score.
The percentile corresponds to z-score -2.0 = 2.28
The percentile corresponds to z-score -0.41 = 34.46
Find the difference between the percentile =
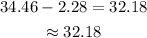
Thus the 18-month-old girl's weight between 19.1 and 23.6 pounds is 32.18%.
Final Answer:
32.18%