The formula that relates the heat and the temperature change is:
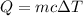
Where Q is the heat, m is the mass, c is the specific heat and ΔT is the temperature change.
Since it absorbes heat, the sign of Q is positive, so:
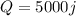
And the change in temperature is:
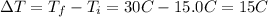
So, solving the equation for m and substituting the values, we have:
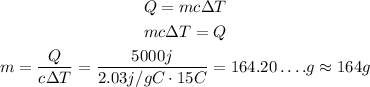
So, the mass of the sample is approximately 164 g.