To calculate the number of molecules we can apply the Avogadro equation, which relates the moles of a compound to the number of molecules. The Avogadro number tells us that in one mole of any substance there are 6.022x10^23 molecules.
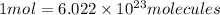
Now we must calculate the number of moles in the mass we are given. For this, we use the molar mass.
The molar mass of CO2 is 44.01 g/mol, so the moles of CO2 in 96.1 g will be:
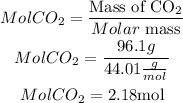
Now we will apply Avogadro to know the number of molecules present in 2.18 moles of CO2.
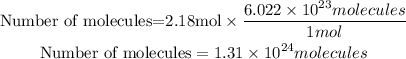
So, in 96.1 g of carbon dioxide, there are 1.31x10^24 molecules