Answer
Q=28.032 KJ
Procedure
To solve this problem, we will consider that we are speaking of liquid water, and we want to calculate the heat to take water from 0 °C to 100 °C without passing from ice to liquid or liquid to gas. Additionally, we are at 1 atm, so the water boils at 100 °C.
Based on the previous, we have the formula
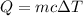
Where,
Q=heat energy
m=mass
c=specific heat capacity
Δ T=change in temperature
Specific heat of water 4184 J/kg°C
Substituting the values
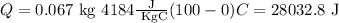
By transforming the previous result into kilojoules by dividing by 1000, we have
Q=28.032 KJ