Answer:
94846.5grams
Explanations
Given the following parameters
molecules of Al2O3 = 5.6 x 10^26 molecules
According to the avogadros constant
1 mole = 6.02 * 10^23 molecules
Convert molecules to moles
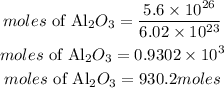
Convert the moles to mass
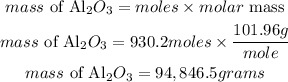
Therefore the mass of 5.6 x 10^26 molecules of Al2O3 is 94846.5grams