Answer:
207.33 kJ.
Step-by-step explanation:
What is given?
Mass of water (m) = 400 g.
ΔT (Change of temperature) = 114 °C - ( - 10 °C) = 124 °C.
Specific heat of water (c) = 4.18 J/g°C.
Step-by-step solution:
To solve this problem we have to use the following formula:
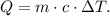
Where Q is heat, m is the mass, c is the specific heat of the water and ΔT is the change of temperature ( T final - T initial).
We want to find the value of Q, so we replace the data that we have:
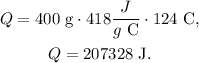
But the problem is asking the answer in kilojoules, so remember that 1 kJ equals 1000 J, so the conversion will look like this:
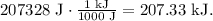
The answer would be 207.33 kJ.