A spectator ion is defined as ions that don't participate in the chemical reaction and just appear on both sides of the equation in the same form:
1. We have to write the equation in its ionic form:
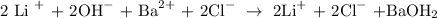
Then, we enlist the repeated ions on both sides of the equation:
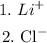
So, the answer is that the spectator ions are Li+ and Cl-.