ANSWER
The mass of NaCl is 292.5 grams
Explanation:
Given information
The number of moles of NaCl is 5.00 mol
Let x represents the mass in grams of NaCl
To calculate the mass in grams of NaCl, we will need to apply the below formula
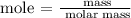
The next step is to find the molar mass of NaCl since we are not given in the question provided
According to the periodic table, the molar mass of sodium is 23 g/mol and the molar mass of chlorine is 35.5 g/mol
molar mass of NaCl = 23 + 35.5
Molar mass of NaCl = 58.5 g/mol
To find the mass of NaCl, we will need to input the given data into the above formula
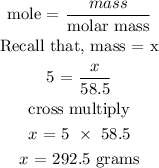
Therefore, the mass of NaCl is 292.5 grams