Explanation and Answer:
a) The ionization reaction of HA in water is given below:
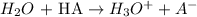
The reaction above occurs between a weak acid and water. A weak acid donates a proton to water. This in turn forms a bronsted base (A-) and hydronium.
b) The conjugate base of HA is A-
The ionization reaction of the conjugate base in water is given below:
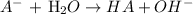
When a base reacts with water, water donates an H+ ion to the base and this in turn froms a weak acid.
Basically, when water reacts with a weak acid, a base is formed. And when water reacts with a base, a weak acid is formed.