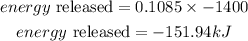Answer:
-151.94kJ
Explanations:
Given the chemical reaction
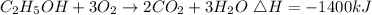
According to the given equation, 1 mole of ethanol will release 1400kJ.
Determine the mole of 5 grams of Ethanol
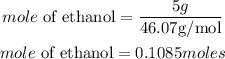
The amount of energy the 0.1085moles of ethanol will release is be expressed as: