You can see that in the reaction, 2 moles of HgO produces 1 mol of O2 and we need to find the number of needed moles of O2 that are decomposed by 19.9 moles of HgO. We can use a rule of three, like this:
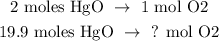
The calculation would be:
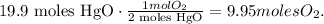
The answer is that 9.95 moles of oxygen are decomposed by 19.9 moles of HgO.