The cases A and C could reach the equilibrium.
The kp equation for the given reaction is:
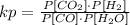
Analyzing each case we see that:
A) Here, regardless of the values, the resut will be equal to 1. So, it is possible to reach the equilibrium state.
B) In this case, depending on the values, the result will be a very large or very small number, different from 1, so it will not be possible to reach the equilibrium state.
C) Same as in casa A, regardless of the values, the result will be equal to 1 and will be possible to reach the equilibrium state.
D) In this case, H2O it is not considered, so it could not reach the equilibrium according to the equation of the reaction.