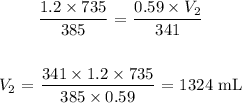Answer:
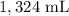
Step-by-step explanation:
Here, we want to get the final volume
Mathematically, from the general gas formula:
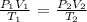
P1 is the initial pressure which is 1.2 atm
V1 is the initial volume which is 735 mL
T1 is the initial temperature which is 112 degrees Celsius (we convert to Kelvin by adding 273: 273 + 112 = 385 k)
P2 is the final pressure which is 0.59 atm
V2 is the final volume which is unknown
T2 is the final temperature which is 68 + 273 = 341 K
Substituting the values, we have it that: