Answer: the pH of this solution is 9.89 and the best option to answer the question is letter B.
Step-by-step explanation:
The question requires us to calculate the pH of a solution, given the hidrogen ion concentration of this solution (1.3 x 10^-10 M).
The pH of a solution can be defined as:
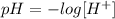
where [H+] corresponds to the hidrogen ion concentration in solution.
Applying the value of [H+] given by the question to the equation above, we'll have:
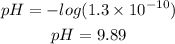
Therefore, the pH of this solution is 9.89 and the best option to answer the question is letter B.