ANSWER
Step-by-step explanation
Given that;
The initial temperature of the gas 280K
The initial volume of the gas is 3.96L
The final volume of the gas is 8.28L
Follow the steps below to find the final temperature of the gas
In the given data, the pressure is fixed and Charles's law is applicable to the system
Charle's law states that the volume of a given mass is directly proportional to its temperature of the gas provided the pressure remains constant.
Mathematically
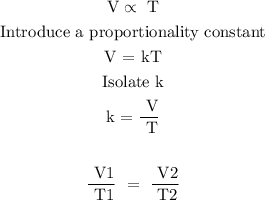
Substitute the given data into the above formula to find the final volume of the gas
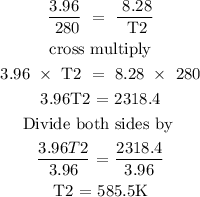
The final temperature of the gas is 585.5K