The half-life of a compound is the time needed to the mass to reduce to half of its initial value. If x is the time in years, x₀ is the half-life or the compound, m₀ the initial mass, m the mass after x years, we can use the following formula:
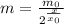
For m = 22 grams, x₀ = 30 years, and x = 11 years, we are looking for the value of m₀. From the first relation, we can isolate m₀ and substitute the values:
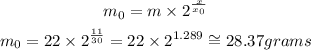
From the present solution, we conclude the answer is c. 28.37 grams.