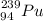First, we need to know the meaning of A, Z, and X.
A: mass number = n + p (neutrons + protons)
Z: atomic number, you can have it from the periodic table, and it gives you the number of electrons or protons.
Remember this: an atom has the same number of protons and electrons.
X: the element.
---------------------------------------------------------------------------------------------------
We have Plutonium-239 (Pu-239)
239 here is its mass number (A)
A= 239 = n + p
From the periodic table, Plutonium has 94 as atomic number.
So, Z = 94 = protons = p
Then, A = 239 = n + p = n + 94 ===> 239 - 94 = 145 = n
----------------------------------------------------------------------------------------------------
Answer:
Plutonium-239
n (neutrons) = 145
p (protons) = 94