1) List the known and unknown quantities.
Sample: air.
Pressure: constant
Volume 1: 157.9 mL.
Temperature 1: 116 ºC.
Volume 2: 62.7 mL
Temperature 2: unknown.
2) Convert units
2.1- Convert mL to L
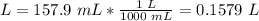
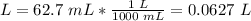
2.2- Convert ºC to K
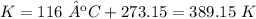
3) Set the equation.
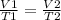
4) Plug in the known quantities and solve for T2.
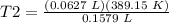
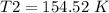
5) Convert K to ºC.
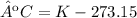
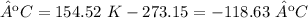
The temperature is -119 ºC.
.