INFORMATION:
We know that:
- a reversible reaction has a forward rate constant of 0.412 mol/L/s
- the reverse reaction rate constant of the reaction is 0.827 mol/L/s
And we must find the equilibrium constant for this reaction
STEP BY STEP EXPLANATION:
To find it, we need to use that:
So, the equilibrium constant is
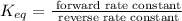
Now, replacing the given information in the formula:
- forward rate constant = 0.412 mol/L/s
- reverse rate constant = 0.827 mol/L/s
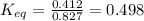
Finally, the equilibrium constant for this reaction is 0.498
ANSWER:
D) 0.498