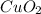The empirical formula is the simplest way to represent a compound. It tells us what the relationship is with respect to the number of atoms between one element and another. To find the empirical formula in this case, we must first calculate the moles that react in the experiment. The number of moles will be found by dividing the given grams by the molar mass of the compound.
Molar mass Cu=63.546g/mol
Molar mass O2=31.998g/mol
Let's see then how many moles react:
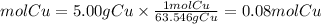
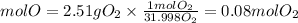
We see that the amount in moles of both compounds are equal. Therefore, the relationship is 1 to 1, that is, for each mole of metallic copper, one mole of gaseous oxygen reacts. So, the produced compound of this reaction will have the following empirical formula: