So,
First, remember that the density is defined as the mass of a substance per unit of volume.
We can express this definition with the following equation:
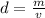
Where d represents the density, m the mass and v the volume.
In this problem, we're given that m=80.0g and v=1.26g/cm3. If we want to find the volume, we can write:
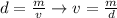
So, we divide:
![\frac{80g}{\frac{1.26g}{\operatorname{cm}3}}=63.49cm^3]()
The answer has to be given in mL, so:
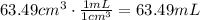
So, the volume is 63.49mL.
With a significant digit, the answer is 63.5 mL.