ANSWER
The partial pressure of carbon dioxide is 0.47 atm
Step-by-step explanation:
Given information:
Total pressure = 2.68 atm
The partial pressure of the oxygen atom = 1.56 atm
The partial pressure of the carbon monoxide = 0.65 atm
Let x represents the partial pressure of the carbon dioxide
Recall that, Dalton's law of partial pressure states that "total pressure is the sum of the partial pressure present in the mixture".
Total pressure = partial pressure of O2 + partial pressure of CO + partial pressure of CO2
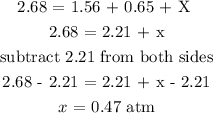
Therefore, the partial pressure of carbon dioxide is 0.47 atm