The reaction:
CH4(g) + 2O2(g) => CO2(g) + 2H2O(g) (written and balanced)
The formula used: rate of CO2
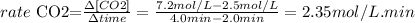
From the reaction is shown the relation between CO2 and H2O produced:
2 H2O : 1 CO2
Therefore, the rate of production of H2O will be the twice of the CO2 rate:
rate H2O = 2 x 2.35 mol/L min = 4.7 mol/L min
Answer: 4.7 mol/L min