Step 1 - Balancing an reading the chemical equation
The provided chemical equation is:
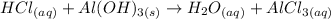
This equation is not balanced yet. The first step in any stoichiometry exercise is to balance the equation:
![3HCl_((aq))+Al(OH)_(3(s))\operatorname{\rightarrow}3H_2O_((aq))+AlCl_(3(aq))]()
The equation is now properly balanced and we can "read" it as:
3 moles of HCl react with one mole of Al(OH)3 thus producing 3 moles of water and one mole of AlCl3
As the exercise is specifically asking about the proportion between HCl and Al(OH)3, we can further simplify this statement to:
3 moles of HCl react with one mole of Al(OH)3