INFORMATION:
We know that:
- there are 2.0L of solution
- the solution has a molarity of 0.50M
And we must find the number of moles of the solute (sodium nitrate)
STEP BY STEP EXPLANATION:
To find it, we need to use the formula of the molarity
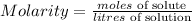
Is given that,
- Molarity = 0.50M = 0.50 mol/L
- litres of solution = 2.0L
Now, replacing in the formula
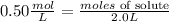
Finally, solving for the moles of solute
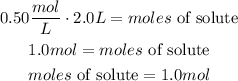
ANSWER:
There is 1.0mol of sodium nitrate in 2.0L of a 0.50M solution