Answer:
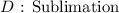
Step-by-step explanation:
Here, we want to get the kind of phase transition that occurred
From the information given, we have a transition from the solid state to the vapor state. However, an important point stood out. There was no liquid phase in the transition
This kind of phase change is what we know as sublimation
It is the direct change of state from the solid to the vapor state of some substances. Other substances capable of this include iodine, naphthalene etc