(3x + 4)(x-1)
Step-by-step explanation:
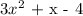
Factors of of the produnct whose sum gives 1:
product = 3(-4) = -12
factors of -12 whose sum gives 1: +4 and -3
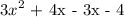
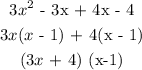
A polynoial is prime if it is an irreducible polynomial- if it cannot be factorised.
Since, the above polynomial was factorsed, it not a prime polynomial.
The factored form:
(3x + 4)(x-1)