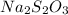The first step to answer this question is to assume that the given percents are masses.
Then, convert these masses to moles using the corresponding molar mass:
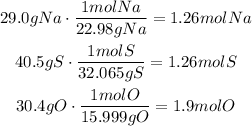
Divide each result by the least result (1.26mol):
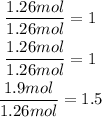
Since not all of these are whole numbers, we have to multiply them times 2:
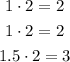
These numbers are the subscripts of each element in the empirical formula.
It means that the empirical formula of this compound is: