Step 1 - Revising the definition of molar concentration (M)
The molar concentration (M) may be defined as:
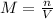
In this equation, n corresponds to the number of moles of solute, whereas V stands for the total volume of the solution.
If we already know the concentration, we can find the number of moles contained in a given volume.
Step 2 - Finding the number of moles
The exercise says the concentration is 3 M and the volume is 10L. Setting these values in the equation:
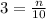
Now, we can find the number of moles n:
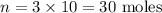
Answer: there are 30 moles of Na2CO3 in 10L of this solution.