Answer:
449.65L
Explanations:
2) According to the Boyle's law, the pressure of a given mass of gas is inversely proportional to its volume provided that the temperature is constant.
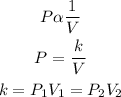
P1 and P2 are the initial and final pressures
V1 and V2 are the initial and final volumes
Given the following parameters
V1 = 509litres
P1 = 697atm
P2 = 789atm
Substitute to determine the new volume V2
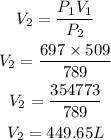
Hence the new volume of the gas is approximately 449.65L