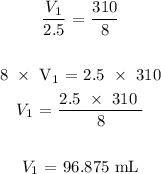Answer:
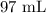
Step-by-step explanation:
Here, we want to get the initial volume of the gas
From Avogadros' law, we know that the volume of a gas is directly proportional to the number of moles of the gas at constant temperature and pressure
Mathematically:
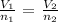
where:
V1 is the initial volume that we want to calculate
V2 is the final volume which is 310 mL
n1 is the initial number of moles which is 2.5 moles
n2 is the final number of moles which is 2.5 + 5.5 = 8 moles
We can now proceed to substitute the values as follows: