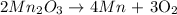Answer
The coefficient of Mn2O3 after balancing = 2
Step-by-step explanation
Given: Mn2O3 ----> Mn + O2
Required: Balance the equation
Left side Right side
Mn = 2 Mn = 1
O = 3 O = 2
Multiply Mn by 4 on the right side, then put 2 infront of Mn on the left side. Now you have 6 oxygens on the left, therefore multiply O by 3 on the right.
Left side Right side
Mn = 2 x 2 = 4 Mn = 1 x 4 = 4
O = 3 x 2 = 6 O = 2 x 3 = 6
Therefore the balanced equation is: