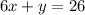Given the slope of the line:
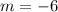
And this point on the line:
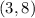
(a) You need to remember that the Slope-Intercept Form of the equation of a line is:
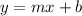
Where "m" is the slope and "b" is the y-intercept.
In this case, you can substitute the slope and the coordinate of the given point into the equation, and then solve for "b":
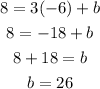
Now you can write the equation of the line in Slope-Intercept Form:
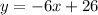
(b) The Standard Form of the equation of a line is:
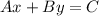
Where "A", "B", and "C" are integers and "A" is positive.
In this case, you need to add this term to both sides of the equation found in Part (a), in order to write it in Standard Form:
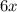
Then, you get:
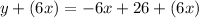
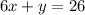
Hence, the answers are:
(a) Slope-Intercept Form:
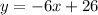
(b) Standard Form: