Let V represents the volume of the gas
Let P represents the pressure of the gas
Let T represents the temperature which is a constant
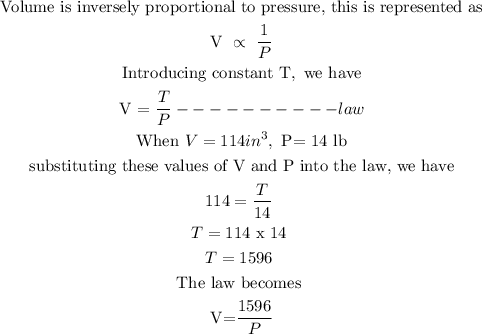
When the volume is decreased to 57 cubic inches, we will have to substitute V=57 into the law and obtain the value of P
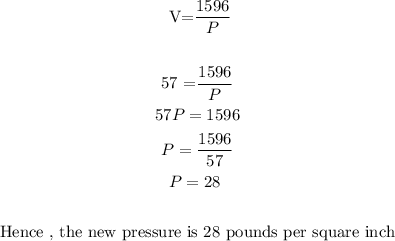
The correct option is option C