The first step to answer this question is to find the number of moles of Ca(NO3)2 that contain 2.50x10^-2 moles of nitrate ion. According to the chemical formula of Ca(NO3)2, there are 2 moles of nitrate ions per mol of Ca(NO3)2:
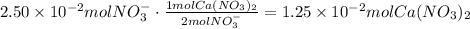
The next step is to use the concentration of the Ca(NO3)2 solution to find the volume that contains this number of moles:
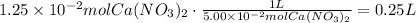
Finally, convert this volume to mL:
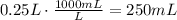
It means that the correct answer is b. 250mL.