Answer: 1.61g of H2O would be necessary to produce 1 L of O2 at STP
Step-by-step explanation:
The question requires us to calculate the mass of water (H2O) that must be decomposed in order to produce 1 liter of oxygen gas (O2) at STP, considering the following chemical reaction:
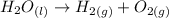
To solve this problem, we'll need to:
1) balance the chemical equation;
2) calculate the molar mass of H2O;
3) calculate the number of moles of O2 that correspond to 1 L of this gas at STP;
4) using the balanced chemical equation, calculate the amount of moles of H2O that would be necessary to produce the required amount of O2;
5) using the molar mass of H2O, determine the mass of H2O necessary to produce 1 L of O2.
1) Balancing the chemical equation
In the given chemical equation, we can see that there are 2 O atoms on the products' side, but only 1 O atom on the reactants side. We can adjust the coefficient of H2O from 1 to 2 in order to have the same amount of O on both sides:
![2H_2O_((l))\operatorname{\rightarrow}H_(2(g))+O_(2(g))]()
Next, we need to adjust the amount of H atoms: there are 4 H atoms on the reactants side, and only 2 H atoms on the products side, thus we can adjust the coefficient of H2 from 1 to 2. The balanced chemical equation for the decomposition of H2O to H2 and O2 can be written as:
![2H_2O_((l))\operatorname{\rightarrow}2H_(2(g))+O_(2(g))]()
2) Calculating the molar mass of H2O
We'll need the molar mass of H2O to determine its required mass, and we can determine the molar mass from the atomic masses of H and O:
O = 15.99 amu
H = 1.008 amu
We can calculate the molar mass of H2O as:
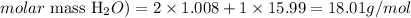
Therefore, the molar mass of H2O is 18.01 g/mol.
3) Calculating the number of moles of O2
Under standard temperature and pressure conditions (STP), 1 mol of any gas corresponds to 22.4 L of this gas. Knowing that 1 L of O2 must be produced, we can calculate the number of moles of O2 as:
22.4 L O2 ----------------------------- 1 mol O2
1 L O2 ----------------------------------- x
Solving for x, we'll have:
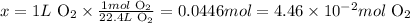
Therefore, 1 L of O2 corresponds to 0.00446 moles of this gas at STP.
4) Calculating the number of moles of H2O necessary
From the balanced chemical equation, we can see the 2 moles of H2O are necessary to produce 1 mol of O2, thus we can write:
1 mol O2 ------------------------ 2 mol H2O
0.0446 mol O2 --------------- y
Solving for y, we'll have:
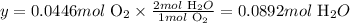
Therefore, 0.0892 moles of H2O would be necessary to produce 1 L of O2 at STP.
5) Calculating the mass of H2O
Now that we know the required amount of H2O, in moles, and the molar mass of H2O, we can determine the mass required of H2O using the following equation:
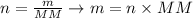
where n is the number of moles, m is the mass of the sample and MM is the molar mass of the compound.
Applying the calculated values of n and M, we'll have:
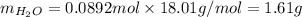
Therefore, 1.61g of H2O would be necessary to produce 1 L of O2 at STP.