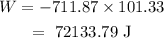Given:
The initial volume of gas is V_i = 122 L
The final volume of gas is V_f = 160.9 L
The pressure of the gas is P = 18.3 atm
To find the work done.
Step-by-step explanation:
The work done in the expansion of gas will be
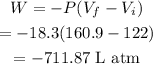
Conversion of L atm to J will be