ANSWER
The final concentration of HCl is 0.0833 M
Step-by-step explanation
Given that;
The initial volume of HCl is 25.0mL
The final volume of HCl is 31.5mL
The initial concentration of HCl is 0.105 M
Apply the dilution formula to find the final concentration of HCl
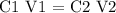
Substitute the given data into the above formula

Therefore, the final concentration of HCl is 0.0833 M