Answer:Ne Volume = 80mL.
Explanations
GIVEN :
• Volume (V1) = 40ml
,
• Temperature (T1)= 20° C
,
• Temperature (T2) = 40°C
,
• Volume (V2) = ?
We will apply Charles law formula : that states that volume is directly proportional to its absolute temperature, if the pressure remains constant
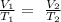
Substituting the given parameters into the above formula, we get that V2 =
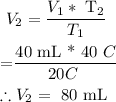
Therefore , the new volume = 80mL