Answer:
The pressure of the gas is 5.8atm.
Step-by-step explanation:
The information given in the exercise is:
- Number of moles (n): 7.2mol
- Volume of the gas (V): 63.5L
- Temperature of the gas (T): 350°C (623K)
We can use the Ideal Gases formula to calculate the pressure of the gas, replacing the values of n, V and T.
Remember that the units must be always in atm, liters, mol and Kelvin when using the Ideal Gases formula:
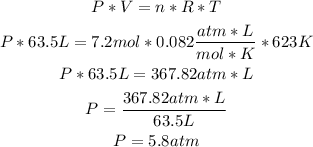
So, the pressure of the gas is 5.8atm.