To answer this question we first look at the reaction:
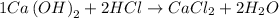
As we can see the equation is balanced, and for every 1 mol of calcium hydroxide and 2 moles of hydrochloric acid we obtain 2 moles of water.
So now we have to calculate how many moles of calcium hydroxide are 29g, and how many moles of hydrochloric acid are 12.5g.
For this we use these compounds molar mass:
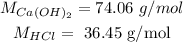
So we calculate the moles of each reactant:
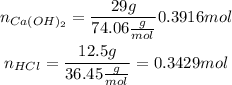
If all the calcium hydroxide reacted then we would need:
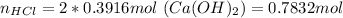
But the moles of hydrochloric acid are 0.3429, therefore the limiting reactant is hydrochloric acid.
Now, to answer the question, we calculate the number of moles formed when 0.3429 moles of hydrochloric acid react.
For every mole of HCl that reacts we said that 1 mol of H2O is formed, so the answer to the question is 0.3429 mol of water can be formed.