The molarity is defined as:
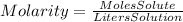
We have the molarity (2.8M) and the volume (23mL). We have to take into account that volume must be expressed in liters, so the volume that we will use will be:
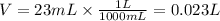
So, the moles of solute will be:
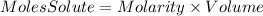
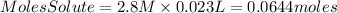
Answer: There are 0.0644moles