Answer:
22.4g of O2 are left over.
Step-by-step explanation:
1st) It is necessary to find out which compound is the limiting reactant and which compound is the excess reactant, so we have to use the stoichiometry of the reaction and the moles given in the exercise:
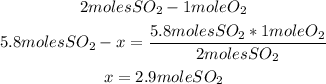
Now we know that 5.8 moles of SO2 will need 2.9 moles of O2 to react properly, but we have 3.6 moles of O2 (more that needed), so O2 is the excess reactant and SO2 is the limiting reactant.
2nd) Initially we have 3.6 moles of O2, but only 2.9 moles will react with the SO2, so we have to substract 3.6 minus 2.9 to calculate the remaining moles of O2:
3.6 moles -2.9 moles = 0.7 moles
3rd) Finally, we have to use the molar mass of O2 (32g/mol) to convert the 0.7 moles to grams:
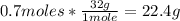
So, 22.4g of O2 are left over.