Okay so if the temperature is constant, and you want either V or T then it is Charles gas law:
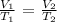
We are given the following:
V1 = 1234 mL
T1 = 237 degrees celsius
V2 = 1023.4 mL
T2 = ?
Since we want the answer in degrees celsius, we do not need to covert to kelvin.
T2 = (T1V2)/V1
T2 = (237 x 1023.4)/1234
T2 = 196.5525122 degrees celsius