The first step to answer this question is to determine the amount of HCl that is reacting:
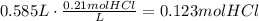
According to the reaction, 2 moles of HCl produces 1 mole of CO2. The next step is to use this ratio to find how many moles of CO2 are produced:
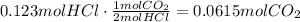
The final step is to use ideal gas law to find the volume of 0.0615 moles of CO2 at the given conditions. Remember that we have to convert the temperature to Kelvin degrees and use the ideal gas constant as 0.082atmL/molK:
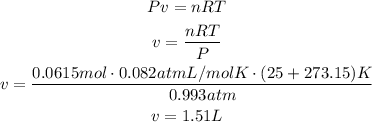
It means that 1.51L of CO2 are produced.