Answer
The limiting reactant is Al
The grams of Al2O3 formed = 90.86 grams
Step-by-step explanation
Given:
Reacting mass of Al = 48.09
Reacting mass of O2 = 51.49
Equation for the reaction:
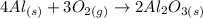
What to find:
The limiting reactant and the grams of Al2O3 formed.
Step-by-step solution:
From the Periodic Table;
Molar mass of Al = 26.982 g/mol
Molecular mass of O2 = 31.998 g/mol
Convert the given reacting mass of Aland O2 to mole to determine the limiting reactant:
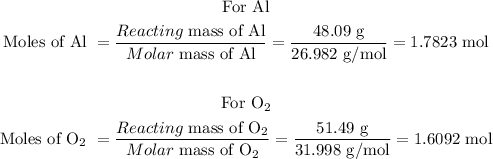
From the balanced equation above; 4 mol Al reacts with 3 mol O2 to form 2 mol Al2O3.
1.6092 mol O2 is expected to react with 2.1456 mol of Al but we have only 1.7823 mol Al. Hence, Al is the limiting reactant.
So, you can calculate the grams of Al2O3 formed from the limiting reactant:
4 mol x 26.982 g/mol = 107.928 g Al formed 2 mol x 101.96 g/mol = 203.92 g Al2O3
1.7823 mol x 26.982 g/mol = 48.090 Al will form:
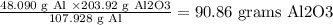
Therefore, the limiting reactant is Al and the grams of Al2O3 formed = 90.86 grams.