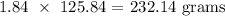Answer:
Step-by-step explanation:
Here, we want to get the mass of manganese chloride
We start by getting the number of moles of HCl
Mathematically:
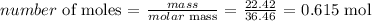
From the information given, 1 mole of Hcl produces 3 moles of MnCl2
Thus, the number of moles of MnCl2 produced will be:
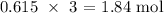
Finally, to get the mass, we multiply the above number of moles by the molar mass of MnCl2
Mathematically, we have that as: