The heat required to raise the temperature of a substance is described by the following equation:
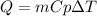
Where,
Q is the heat required to raise the temperature, 4485 J
m is the mass of the substance, 100g
Cp is the specific heat
deltaT is the temperature difference
We must clear the specific heat from the equation and substitute the known values.
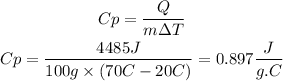
The specific heat of aluminium will be 0.897J/g.°C