Answer:
a. 0.16 (M).
Step-by-step explanation:
What is given?
Volume 1 (V1) = 15.8 mL,
Concentration 1 (C1) = 7.5 M,
Volume 2 (V2) = 743.9 mL.
What do we need? Concentration 2 (C2).
Step-by-step solution:
To solve this problem, we have to use the following formula:

Where C indicates concentration and V indicates volume.
We want to find C2, so let's solve for this unknown value and replace the given data in the formula:
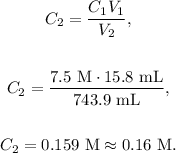
The answer would be a. 0.16 (M).